Good Lay Summary Practice
Introduction
Attention, clinical researchers! If you think a lay summary is just a simplified translation of your research findings, think again! A lay summary is much more than that. It is a powerful tool that helps you communicate your research in a way that is accessible and engaging to a wider audience.
What is a Good Lay Summary


A good lay summary does not just break down complex scientific jargon into simpler terms – it also highlights the most important aspects of your research and presents them in a way that is relevant and meaningful to the general public. This means considering the context of your research, the potential impact it could have, and how it fits into the larger scientific landscape.
By taking the time to craft a well-written lay summary, you are not only making your research more understandable to non-experts, but you are also contributing to a more inclusive and collaborative scientific community. So, do not just settle for a dry, technical summary – put in the effort to create a lay summary that truly captures the essence of your research and inspires others to learn more about your field.
The “good” lay summary is a recommended way of creating these summaries, which is the backbone of the Good Lay Summary Practice (GLSP) Guideline.
Bridge the gap between science and the people it serves
A good lay summary should put the patient at the centre, highlighting how your research could potentially improve their quality of life, alleviate symptoms, or lead to new treatments. By using clear and accessible language and framing your findings in terms of real-world outcomes, you can help patients better understand the significance of your work and how it could potentially affect their lives.
So, do not just think about your research in terms of abstract concepts and technical details. Keep the patient in mind at every step of the process, and use your lay summary as a way to communicate the real-world impact of your work to the people who matter most. By doing so, you will be contributing to a more patient-centred approach to research and helping to bridge the gap between science and the people it serves.
How should a Good Lay Summary (GLS) be


A GLS needs to be understandable
Clinical researchers, if you want your work to have an impact beyond the scientific community, you need to make it accessible to non-experts. A lay summary is your chance to break down the technical language and jargon of your research and communicate your findings in a way that anyone can understand.
It is important to remember that a GLS is not a tool for decision-making when it comes to changing or starting therapy. Instead, it should provide understandable and valuable information on the context of your trial in the drug development process and emphasize the need for further research before any treatment decisions can be made with confidence.
A GLS should avoid oversimplifying information so much that the opportunity to educate patients about a particular disease area gets lost. It should use words that are familiar to a lay audience and technical terms should be explained. Formatting and design are important to making the summary easier to understand: such as in the font size, colour, use of bullet points, and illustrative graphics, charts, or tables.
A GLS needs to be relevant
Clinical researchers, if you want your work to resonate with a wider audience, you need to make it relevant to their lives. A lay summary is not just a summary of your research – it is a chance to highlight the practical applications and real-world implications of your work in a way that is meaningful to non-experts.
A good lay summary should focus on the broader implications of your research and the building of scientific knowledge. By framing your findings in terms of real-world problems and solutions, you can help your audience understand why your research matters and how it could potentially benefit them.
We understand that clinical researchers like you conduct valuable research to gain scientific knowledge and refine methodologies before conducting trials. However, it is crucial to go beyond just sharing trial outcomes and provide the general public with a deeper understanding of the broader research context and the significance of your study.
A GLS needs to be accessible
Attention, clinical researchers! It is time to take note of an important new requirement in clinical research.
Since 31 January 2023, all clinical trial applications in the European Union (EU) for medicinal products must be submitted via the Clinical Trials Information System (CTIS).
Trials authorized under the former Clinical Trial Directive, however, can be continued under the Directive if they finish before 30 January 2025.
CTIS is now the single-entry point for sponsors of clinical trials, regulatory authorities and ethics committees for the submission and application assessment of clinical trials.
It is structured in two restricted and secured workspaces (sponsor workspace and authority/ethics committee workspace) and a public portal.
So, do not wait – make sure you are up to date on the latest requirements for publishing a lay summary in the CTIS. By doing so, you will be helping to promote a more patient-centred and transparent approach to clinical research, and making a real impact in the lives of those who stand to benefit from your work.
Why is a Good Lay Summary (GLS) important
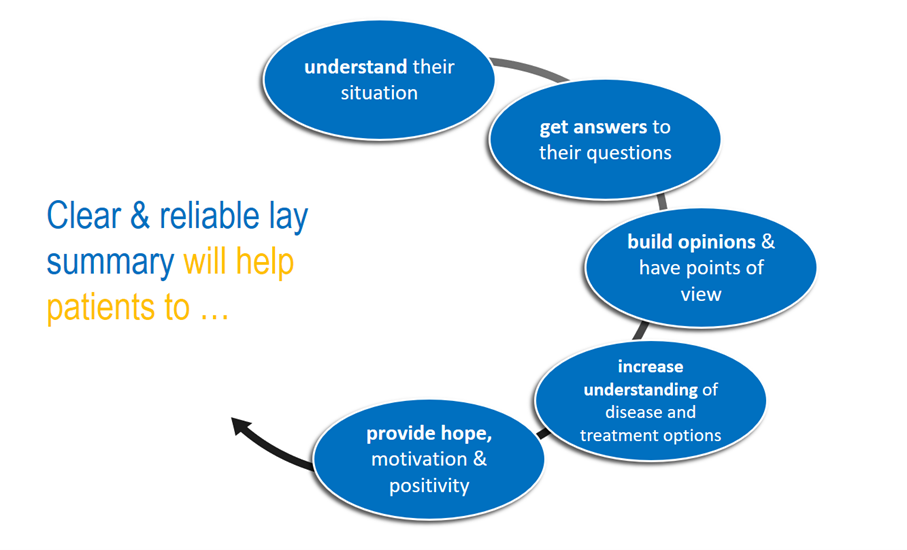

A GLS is a powerful win-win tool for both patients and clinical researchers
Clinical researchers do not underestimate the power of a good lay summary – it is a win-win tool for both patients and clinical researchers. By using this tool to communicate the relevance and impact of your work to the public, you can help promote a more patient-centred and collaborative approach to research, and ultimately make a real difference in the lives of those who stand to benefit from your findings.
For patients, a well-written lay summary can help them better understand the relevance and importance of your research to their illnesses. By using clear and accessible language and highlighting the practical applications of your work, you can help patients feel more empowered and informed about the latest developments in your field.
Why is a Good Lay Summary (GLS) important - Part 2
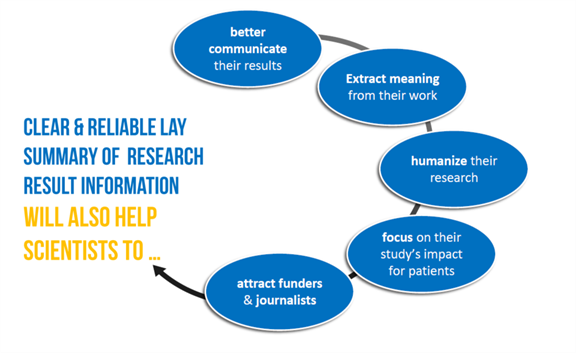

For clinical researchers, a good lay summary can help you bridge the gap between science and the general public, increasing the visibility and impact of your work beyond the scientific community. By communicating your findings in a way that is understandable and relevant to non-experts, you can help build trust and support for your research, and ultimately contribute to a more engaged and informed public.
If lay summaries are shared on social media platforms and other online channels, your research will be more accessible to a wider audience. This can lead to increased engagement and interest in the research and potentially to people being more open to participating in trials.