GLSP Language Toolbox
TRANSLATION PROCESS FOR LAY SUMMARIES
The process below is a recognised gold standard for language translation of information intended for study participants in clinical studies, for example Informed Consent Forms. It is also the recommended translation process in the Good Lay Summary Practice (GLSP) Handbook(1).
The process and scope of language translations for Lay Summaries is determined by the study Sponsor, and the Sponsor is recommended to plan translations already during the development of the Informed Consent Form.
Lay Summaries are written for the study participants and the general public. Since it is imperative that the summaries remain understandable and non-promotional after translation, the GLSP Handbook recommends the gold standard translation process as the ideal setup. The process involves different qualified and independent translators and a forward and back translation step. In case of Sponsor resource constraints, back translation and subsequent comparative review can be replaced by a linguistic review if the Sponsor deems it acceptable from a quality and risk perspective.
For more guidance, refer to the Good Lay Summary Practice (GLSP) Handbook at EudraLex, Volume 10, Clinical trials guidelines, Chapter V.
Translation Process Overview Diagram
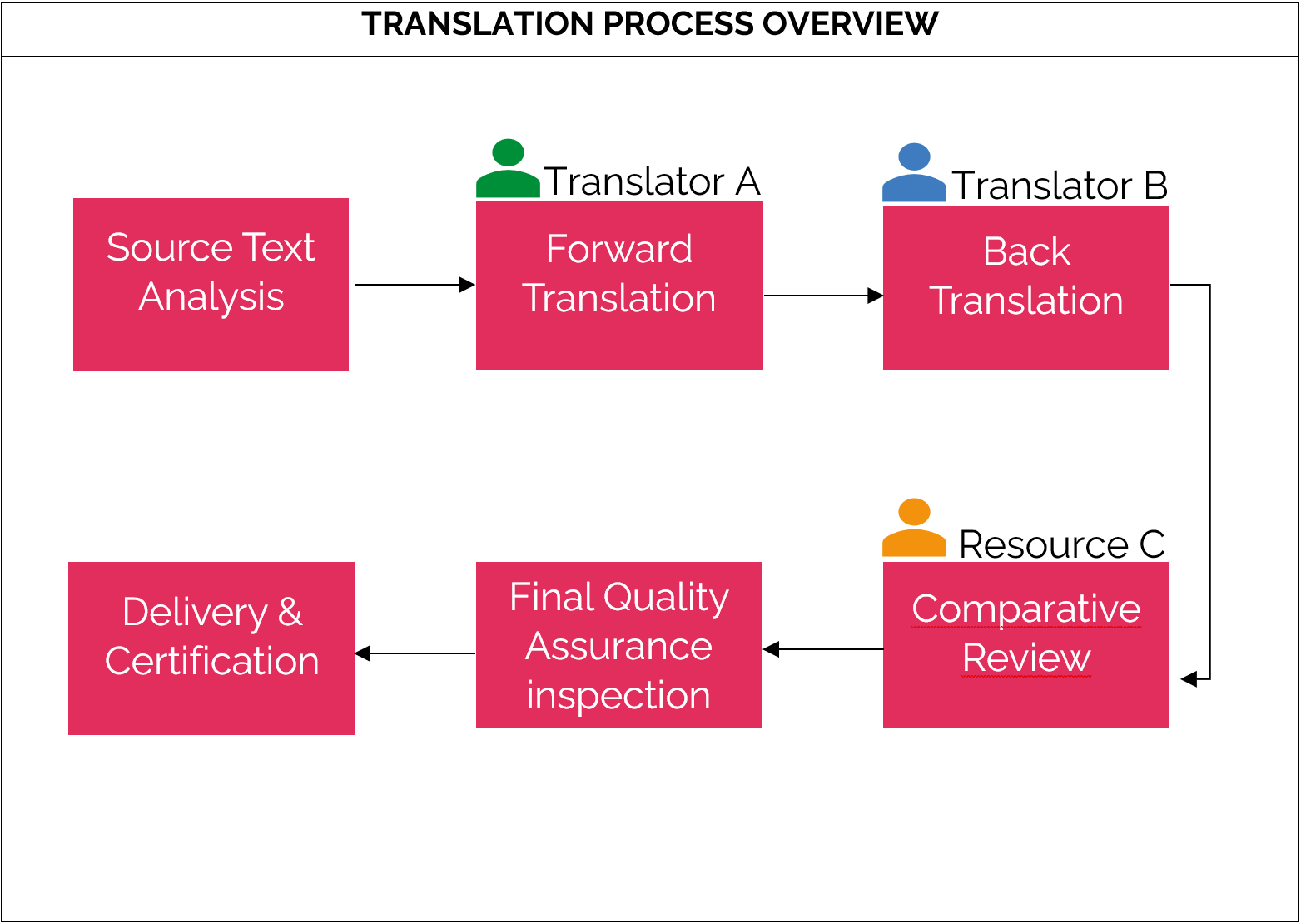
| STEP-BY-STEP TRANSLATION PROCESS DESCRIPTION | ||
1 SOURCE TEXT ANALYSIS | WHAT | WHO |
| Before language translation is initiated, the source text (master Lay Summary) should be analysed in order to identify potential areas of ambiguity, compliance or risk areas of promotional or biased language. This will facilitate decisions on glossaries, reviews or tools needed in the translation process. | Source text analysis is typically performed by a linguist but may be performed by someone else who is trained in the Lay Summary process and understands lay Summaries/ clinical trials. | |
2 FORWARD TRANSLATION | WHAT | WHO |
| Forward translation is the process of translating a source text (master Lay Summary) into a target language (or languages, depending on the language scope determined by the Sponsor). | Forward translation is performed by a qualified translator who is a native speaker or fluent in the target language and who has experience in the medical field/with clinical trials. | |
3 BACK TRANSLATION | WHAT | WHO |
Back Translation is the process of translating a target text (the result of the forward translation) back into the original source language. Back translation is a quality assurance step which is followed by a comparative review. | Back translation is performed by a qualified translator who is a native speaker or fluent in the source language and who has experience in the medical field/with clinical trials. The back translator will not have access to the source text (master Lay Summary) but only to the translated Lay Summary. | |
4 COMPARATIVE REVIEW | WHAT | WHO |
Comparative review is the process of comparing the back translated Lay Summary with the source text (master Lay Summary). Comparative review can help detect and resolve any discrepancies between the source text and the back translation with the intent to arrive at the best possible translation. | A third resource (not necessarily a translator) will perform the comparative review. The comparative reviewer will have access to the source text (master Lay Summary) and the back translated Lay Summary. | |
5 FINAL QUALITY ASSURANCE INSPECTION | WHAT | WHO |
| A final, thorough quality inspection is recommended if DTP (Desktop publishing) or other production quality steps have been included as part of the final file production. | This step can be performed by an individual who is trained in the process. | |
6 DELIVERY & CERTIFICATION | WHAT | WHO |
| The final output is the translated Lay Summary(ies) along with any translation certificates, as applicable. | This step can be performed by an individual who is trained in the process and who can verify that qualified translators were used in the translation process. | |
Health Literacy Principles Non-Promotional Language for Lay Summaries
A recommendation for the development of Lay Summaries is that they are written based on Health Literacy Principles in order to facilitate that the summaries are understandable for a general audience from the age of 12 years. These principles can in addition be a useful guidance for any subsequent language translations of Lay Summaries. Another good practice for Lay Summaries is that they are written in neutral, non-promotional language to avoid any unintended or biased interpretation of clinical study results. Core elements of Health Literacy and Non-promotional language are extracted below from the Good Lay Summary Practice (GLSP) Handbook(1).
| HEALTH LITERACY PRINCIPLES | |
| PRINCIPLES | EXAMPLES/ELABORATION |
| Use simple, everyday conversational language | Use not Utilise Long term not Chronic |
| Use short words, sentences, and paragraphs | To increase readability, it is recommended to use:
|
| Use active voice rather than passive voice | Active voice is easier to understand. It reduces the risk of misinterpretation and can make sentences shorter. Example: "Researchers studies the effect of tamoxifen", rather than "The effect of tamoxifen was studies by researchers." |
Do not use technical or scientific language (jargon) | "Birth control", not "Contraception" "High bood pressure", not "Hypertension" |
| Present medical terms in brackets | Present medical terms in brackets after the plain language version. Example: "Some people had side effects of feeling sick (nausea)". |
| Do not use statistical terms | Do not use terms such as number needed to treat, odds ration and confidence interval |
| Quantify terms | Quantify words such as low, higher, faster, more, many, and ensure content is evidence-based. Example: "Most were non-smokers (44) or former smokers (11)." |
| Use words and terms consistency | Do not alternate between interchangeable synonyms which will be more demanding for the reader. Example: study versus trial |
| Be respectful in your language | "People with cancer" rather than "cancer patients" |
| Do not use Latin expressions | Such as not e.g., That means not i.e., In the laboratory not in vitro |
| NON PROMOTIONAL LANGUAGE | |
| PRINCIPLES | EXAMPLES/ELABORATION |
| Keep an overal factual and objective style/ton |
|
| Avoid commercial or marketig appearance |
|
| Avoid superlative and enthusiastic words |
|
| Quantify statements |
|
| Clarify that results are from a single study only |
|
| Use high level statement with caution |
|
| Ensure that additional information is readily available |
|