Good Lay Summary Practice
You want to make sure your lay summary is effective, impactful, and truly patient-centred Then it is time to adopt good practices and involve patients into the process from the beginning.
Remember, good practice for lay summaries is about more than just meeting legal requirements &ndash it is about creating a document that truly serves patients and the public. So, do not wait &ndash start involving patients from the beginning and make your lay summary the best it can be.
Involve patients from the beginning
By involving patients in the development of your lay summary, you can ensure that it speaks directly to their needs and concerns, and provides them with the information they need to better understand new treatment options – their progress and their complexities. That can ultimately help patients to make informed decisions about their healthcare.
Start by engaging patients early in the process. Ask them what information is most important to them, what questions they have, and what language and format works best for them. Use their input to guide the development of your summary and make sure it meets their needs.
Throughout the process, continue to involve patients and seek their feedback. This will help you identify areas that need improvement and ensure that your summary remains patient-centred and effective.
A consistent approach planning, developing and writing, translation and dissemination
If you want your work to be accessible and impactful for patients and the public, sponsors need a consistent approach for lay summaries across all studies, a dedicated team including plain language experts, and to keep patient involvement integrated in their strategies for lay summaries: planning, developing and writing, translation, and dissemination.
By following these steps, you can ensure that your lay summary is effective, accessible, and impactful for patients and the public. So, do not wait – start planning, developing, translating, and disseminating your lay summary today!
Planning, developing and writing, translation, and dissemination
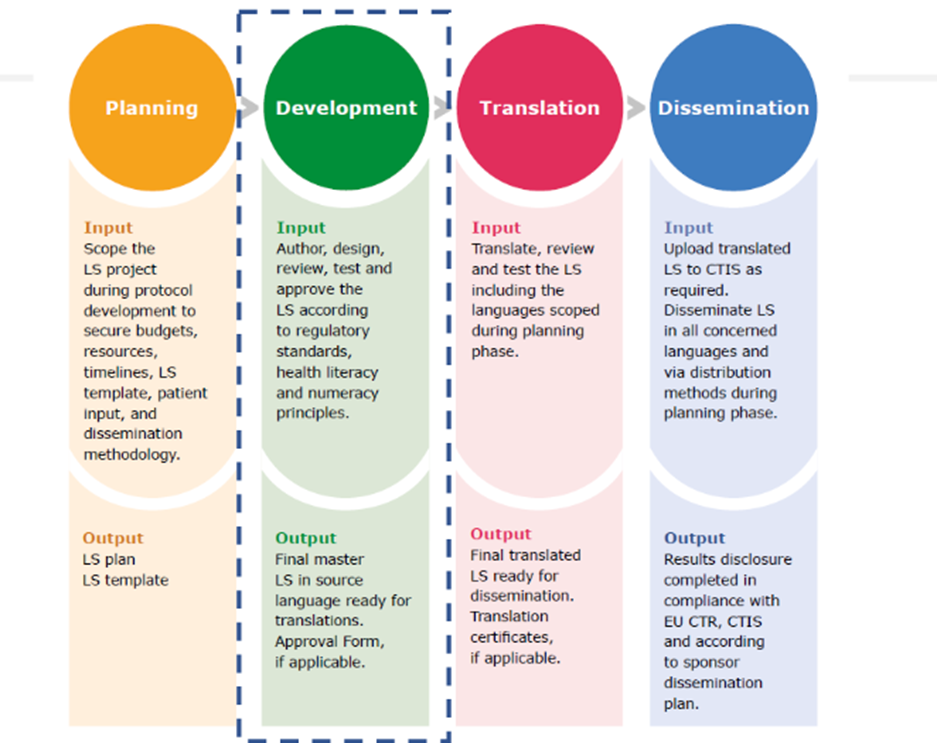

Planning
First, you need to plan out your approach. Who is your audience? What are the key messages you want to communicate? What information is essential to include, and what can be left out? Answering these questions will help guide your development process and ensure that your summary is focused and effective.
- Plan for the entire lay summary process and create a Standard Operating Procedure (SOP) that covers planning, development and writing, translation and dissemination of lay summaries
- Develop a general template for lay summaries that includes graphics and icons
- Provide budget and resources for the production of lay summaries including cost of translation and dissemination
- Develop a strategy for a comprehensive involvement of patients in the production of lay summaries
Development and writing
Next, you need to develop your summary. This means writing a clear and concise summary of your work that is understandable to non-experts. Use plain language, avoid jargon, and highlight the practical applications of your research to help engage and inform your audience.
- Adopt the GLSP guidance for the content of lay summaries
- Establish a team of lay summary writers with the appropriate skills:
- Knowledge of clinical research, scientific knowledge, guidance on lay summaries
- Communication skills, lay language writing and editing
- Translation skills and user testing expertise
- Visual design skills, good graphic design principles
- Consider health literacy and numeracy limitations in the audience(s) in the presentation of text and data in lay summaries
- Develop a consistent approach to and practice of patient involvement
- Ensure that the content and presentation of data is balanced and strictly non-promotional
- Apply appropriate design to make lay summaries attractive
- Use headings and subheadings
- Adequate use of white space
- Develop simple and clear graphics: 1 message per graph
- Prepare reviewers of lay summaries for the task:
- Provide training on purpose and design
- Give reviewers clear guidance on the objective of their review
- For paediatric studies, consider how you will develop lay summaries for children
Translation
Once you have developed your lay summary, it is time to translate it into different languages. This is important if you want to reach a wider audience and make your work accessible to non-English speakers. It is recommended that you translate at least into all the languages into which the Patient Information Sheet and Informed Consent Form were translated, to serve the same people, the same participants in the trial.
- Consider translations as a core activity in the provision of lay summaries
- Plan translations early in the development of lay summaries:
- Consider the Patient Information Sheet and the Informed Consent Form as sources for lay summaries, thus as related lay language documents
- Ensure that translations are accurate and in a language that is considered adequate by (local) readers
- Develop translation memories for certain languages
- Consider process of back-translation and local user-testing of lay summaries to ensure optimal quality and cultural appropriateness
Dissemination
Finally, you need to disseminate your lay summary. In addition to uploading it to the CTIS, you can share it widely through your networks and social media channels.
- Uploading the lay summaries in one EU language into the EU portal is compulsory!
- Sponsors are encouraged to develop other dissemination pathways and plan them carefully
- Optional dissemination methods include:
- Direct dissemination via the investigator to the trial participants (e.g., via postal service or email)
- Indirect dissemination via a public website or patient organisations
- Combination of indirect and direct dissemination
Develop a strategy for comprehensive Patient Involvement in Lay Summary production
If you are looking to develop a comprehensive strategy for involving patients in the production of lay summaries, we have you covered! By involving patients in the production of lay summaries, you will enhance the relevance and impact of your research.
Here are some practical steps to consider:
- Embrace patient perspectives: Recognise the invaluable insights patients bring to the table. Emphasize their role in shaping meaningful lay summaries that resonate with the intended audience.
- Engage patients from the start: Involve patients in the planning and development stages. Collaborate with patient representatives and advocacy groups to ensure diverse voices are heard. Together, co-create lay summaries that truly reflect patient needs.
- Foster open communication: Establish clear channels for ongoing dialogue with patients. Encourage feedback, address concerns, and keep patients informed throughout the process. Creating an inclusive and supportive environment is key to successful collaboration.
- Keep it simple and accessible: Prioritise plain language to make the lay summaries easily understandable for a wide range of readers. Involve patients in reviewing and refining the content, ensuring clarity and accessibility without sacrificing scientific accuracy.
- Provide training and support: Offer resources and training to patients interested in participating in lay summary production. Equip them with the necessary skills to effectively communicate scientific concepts to non-experts. Empowering patients enhances their confidence and enriches the collaborative process.